What federal health agency cuts mean for tobacco control
On April 1, 2025, the U.S. Department of Health and Human Services (HHS) announced the elimination or significant reduction of staff at two essential agencies that regulate tobacco products, monitor tobacco use, and provide resources to quit.
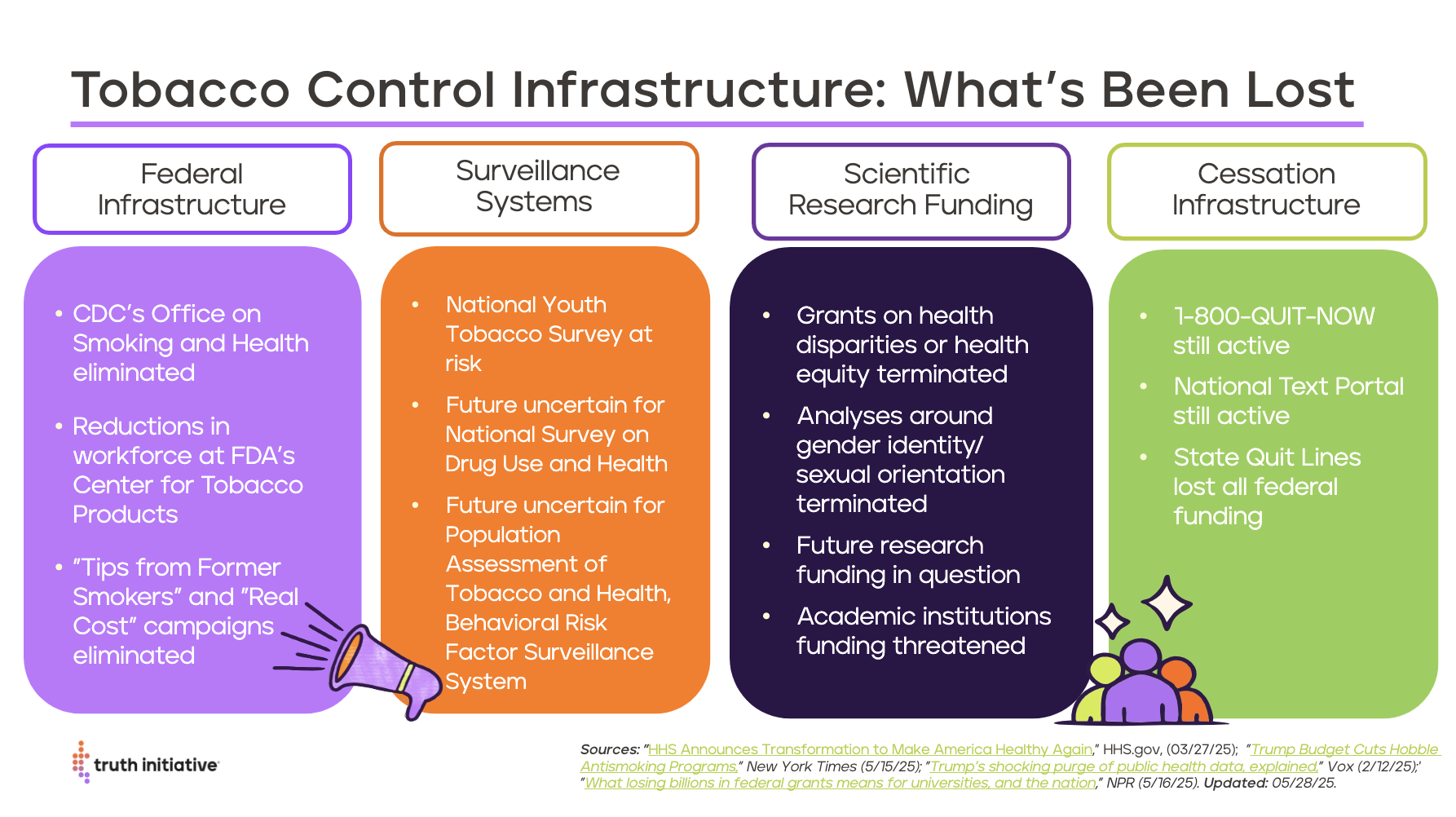
The entire Office on Smoking and Health at the Centers for Disease Control and Prevention (CDC) has been removed, and many staff members at the Center for Tobacco Products at the Food and Drug Administration (FDA) have been placed on administrative leave as part of President Trump’s implementation of the Department of Government Efficiency. These cuts will have a profound impact on federal tobacco control efforts and will likely result in more tobacco products coming on the market and almost no new regulations issued to reduce tobacco-related death and disease.
To share these developments and more, Truth Initiative has created a resource which will track changes in the federal agency landscape and the expected impact on tobacco control measures. This resource will be updated as needed – sign up for our newsletter to receive updates straight to your inbox.
See below for the latest updates related to federal health agency cuts and the impact on tobacco control, and stay up to date by visiting our federal cuts webpage.
The elimination of The CDC Office on Smoking and Health puts public education, data collection, and quitting resources at risk
The Office on Smoking and Health (OSH) at the CDC plays a critical role in preventing youth tobacco use and helping people quit. All CDC OSH staff were laid off during the April 1 cuts. As of May 30, due to a preliminary injunction issued by a federal district judge, the Trump administration is pausing the layoffs at CDC, including the Office on Smoking and Health. Those affected will remain on paid administrative leave until further notice.
The layoffs, extended administrative leave, and potential elimination of CDC OSH will put many tobacco control and cessation programs at risk. CDC OSH provides funding to state health departments for tobacco control efforts, including state quitlines and public education programs such as the Tips from Former Smokers® campaign, which has helped over 1 million people quit and saved states $55 for every $1 spent, mostly in averted health care costs to treat smoking-related illness.
As of May 21, loss of federal funding from CDC OSH has also resulted in:
- The scheduled closure (summer 2025) of a youth-led tobacco prevention program in West Virginia.
- New York State has laid off 13 tobacco control program staff.
- North Carolina’s tobacco prevention and control program has indefinitely furloughed nine staff, leaving only three state-level employees to continue tobacco prevention efforts. This could limit quitline services, including those that provide nicotine replacement therapy (NRT) for uninsured and underinsured North Carolinians, and scale back Tobacco-Free NC initiatives.
The agency also funds data collection, including the National Youth Tobacco Survey, which reports on youth access and use of tobacco products and provides a crucial foundation for policies that protect the health of young people.
Reductions in workforce at the FDA Center for Tobacco Products will likely jeopardize enforcement of current regulations, and limit new ones
The FDA Center for Tobacco Products (CTP) regulates the manufacturing, marketing, and distribution of tobacco products and educates the public about the harms associated with using tobacco. FDA CTP is completely funded by tobacco user fees collected from manufacturers and importers of tobacco; therefore, reductions in workforce have no associated savings for American taxpayers.
As of April 1, layoffs at the agency include CTP Director Brian King, leadership at the Office of Science, (responsible for the review of tobacco products) and leadership and staff working on public education programs. Staff responsible for issuing warnings letters and penalties to retailers selling tobacco products to underage people were laid off but have since been asked to temporarily return.
As of May 9, FDA CTP has also paused compliance checks to ensure tobacco products are not sold to minors and has paused review of premarket applications of tobacco products.
With fewer staff to enforce current regulations and advocate for new ones, these cuts will likely contribute to the continued flow of unregulated tobacco products coming on the market, with very few, if any, new regulations issued to combat illegal, youth-appealing, and highly addictive products.
This article shares updates as of June 5, 2025.
More in tobacco prevention efforts
Want support quitting? Join EX Program
By clicking JOIN, you agree to the Terms, Text Message Terms and Privacy Policy.
Msg&Data rates may apply; msgs are automated.