E-cigarette products sold on the market quadruple in just one year
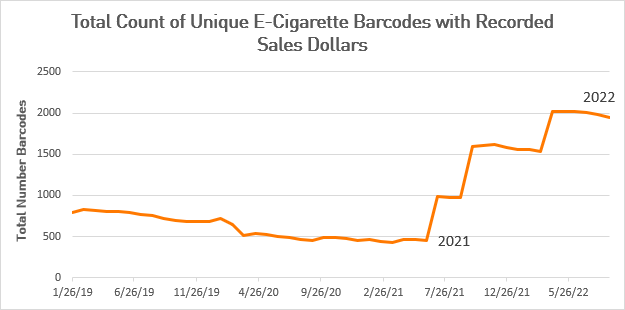
The number of unique e-cigarette products, many of which come in youth-appealing flavors, more than quadrupled from 453 in June 2021 to 2,023 in June 2022, according to a Truth Initiative review of U.S. retail sales data. The increase, based on retail sales estimates from NielsenIQ,* shows how companies continue to flagrantly flout the regulatory process and launch new products without authorization from the U.S. Food and Drug Administration (FDA), a requirement to legally enter the market.
As youth e-cigarette use remains a serious public health threat, with over 2.5 million middle and high school students reporting current e-cigarette use in 2022, the proliferation of unauthorized e-cigarettes underscores the urgent need for comprehensive regulation and enforcement.
The state of e-cigarette regulation and enforcement
FDA vaping regulations
After years of delays, the FDA is undergoing its review of pre-market tobacco applications (PMTA) from e-cigarette makers to determine whether their products can stay on the market. Any products that had not received authorization by September 2021 were supposed to have been removed and no new products are allowed to enter the market without pre-authorization. Yet the tobacco industry continues to flood the market with new products and apparently little fear of federal reprisal.
To date, less than 30 e-cigarette products have been granted marketing authorization by the FDA, covering a small slice of the e-cigarette market that does not include the products most popular with youth. Decisions remain pending on applications from leading e-cigarette brands such as JUUL, Vuse (Alto), blu, and others that make up between 65% and 70% of the market and are among the most popular with youth. In the meantime, the FDA issued marketing denial orders to two Reynolds menthol e-cigarette products in January 2023, and in 2022 to Logic, which makes up just over 1% of the e-cigarette market according to retailer scanner data. The recent acquisition of popular e-cigarette brand NJOY by Altria also raises concerns that the tobacco company will use its vast marketing and distribution systems to increase NJOY’s reach and availability.
The FDA says it has acted on 99% of the 6.7 million applications received, and more than 260 e-cigarette companies that submitted a PMTA have received marketing denial orders. This should translate to fewer e-cigarette barcodes with recorded sales dollars. Instead, NielsenIQ data clearly show a sharp increase in the number of unique e-cigarette product barcodes with sales peaking at 2,023 products in June 2022. Although data indicate that the number of unique products has leveled off recently, there were still nearly 2,000 e-cigarette products with sales in September 2022, the most recent data reviewed.
Urgent need for comprehensive regulation of e-cigarettes
what is the new law for vaping
The rapid rise of e-cigarette products has the effect of overwhelming the FDA. The flood of new e-cigarette products creates an even greater back log of PMTAs for the FDA.
The FDA has acted in the past to address the situation. For example, in October 2022, the FDA filed complaints for permanent injunctions against several e-cigarette manufacturers through the U.S. Department of Justice. In February of 2023, the FDA also announced it filed civil money penalty (CMP) complaints against four tobacco product manufactures for making and selling e-cigarette liquids without FDA authorization.
The Center for Tobacco Products (CTP) recently shared concrete steps that may ultimately help prevent tobacco manufacturers from playing catch-me-if-you-can with the FDA. CTP announced its intention to develop and implement a five-year strategic plan by December 2023, which will help the agency pivot from a reactive mindset to a proactive one. Truth Initiative underscores the need for this plan to include a clear, scientifically sound, and executable strategy to regulate and enforce the continued proliferation of youth-appealing tobacco products. The agency pledged commitment to greater transparency about its compliance and enforcement, promising to publicly share a list of legally marketed products. The agency also announced that it will work with other federal agencies to enhance their enforcement work.
It is critical for the FDA to pursue these efforts expediently to protect our nation's young people from companies that continue to thwart the law and put a new generation of young people at risk for nicotine addiction.
*The conclusions drawn from the NielsenIQ data are those of the researchers and do not reflect the views of NielsenIQ. NielsenIQ is not responsible for, had no role in, and was not involved in analyzing and preparing the results reported.
More in emerging tobacco products
Want support quitting? Join EX Program
By clicking JOIN, you agree to the Terms, Text Message Terms and Privacy Policy.
Msg&Data rates may apply; msgs are automated.