Truth Initiative’s Quit Vaping Program Effective in Helping 18- to 24-Year-Olds
Clinical trial shows This Is Quitting increased e-cigarette quit vaping rates by nearly 40%
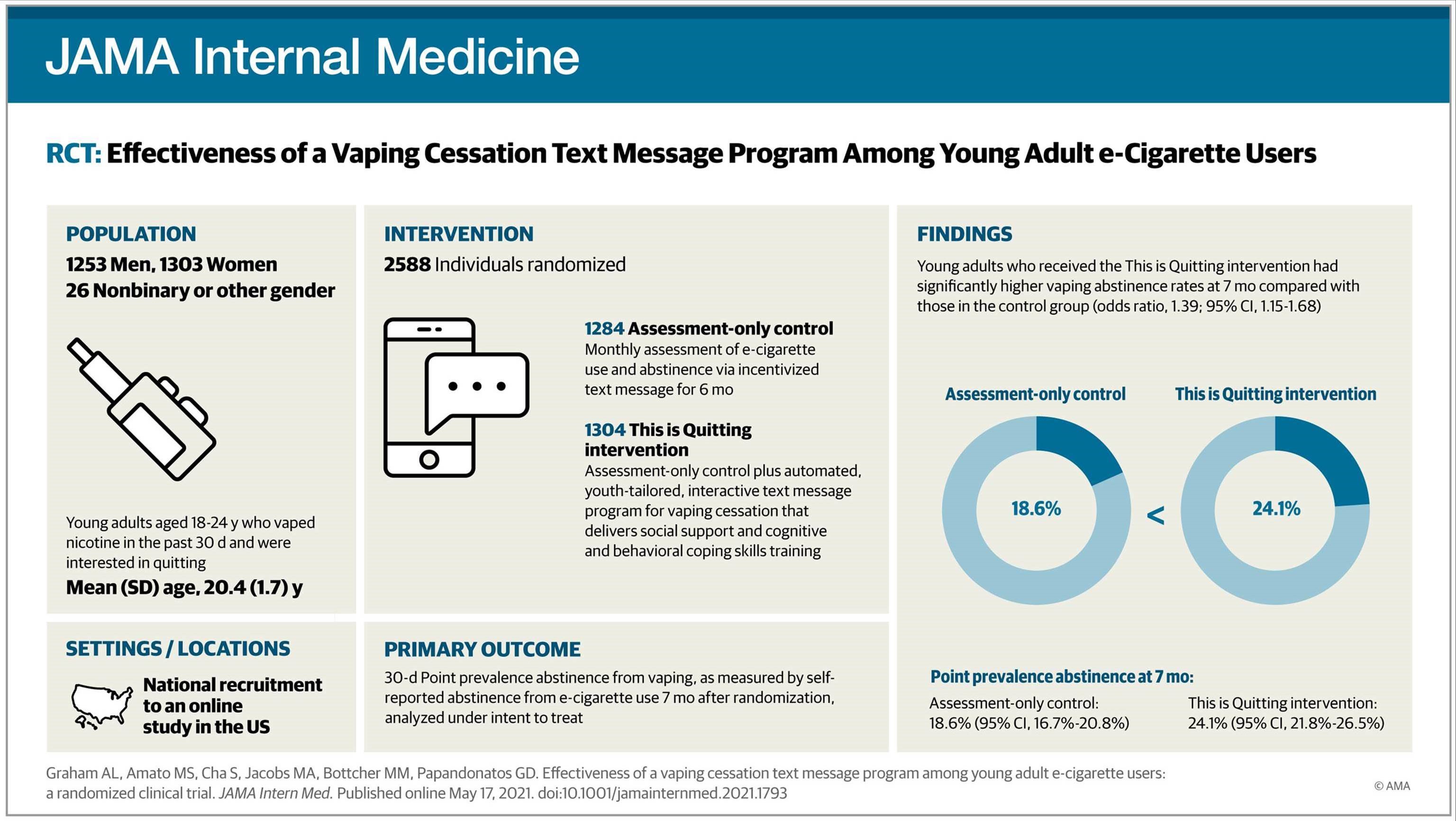
A new study published on Monday in JAMA Internal Medicine demonstrates the effectiveness of Truth Initiative’s This is Quitting – a free and anonymous quit vaping text message program – in helping young adults to quit vaping e-cigarettes. The randomized clinical trial found that young adults aged 18-24 who used This is Quitting had nearly 40% higher odds of quitting compared to a control group. The results were consistent across all characteristics examined in the randomized trial, including demographics, nicotine dependence, social influences to vape, mental health symptoms, and other substance use.
These data come at a time when e-cigarette use among young people remains at epidemic levels, and public health organizations have called for research on e-cigarette cessation programs. The 2019 CDC National Health Interview Survey found that 9.3% of young adults ages 18-24 used e-cigarettes, up from 7.6% in 2018, and over half (56%) of the young adult e-cigarette users reported that they had never smoked cigarettes. The nicotine found in most e-cigarettes can expose young people to negative health effects around brain development – which continues up to age 25 – including an increased risk of nicotine addiction and mood disorders. A separate Truth Initiative survey found that 60% of the young e-cigarette users surveyed, aged 15-24, want to quit within the year and of those who want to quit, more than half (51.2%) agreed that texting would help them stop vaping, underscoring the urgent need for effective programs like This is Quitting.
Launched in January 2019, This is Quitting was created specifically for teens and young adults looking to quit e-cigarettes. The interactive text message program has enrolled over 315,000 young people to date, providing them with tailored advice, cognitive and behavioral coping strategies, and social support to help them quit. This is Quitting is now an integral part of the truth® campaign, Truth Initiative's proven-effective national youth smoking, vaping and nicotine prevention public education campaign. Young people can text “DITCHVAPE” to 88709 to enroll.
“Free and tailored resources that help young people quit vaping are vital in the fight against the ongoing youth e-cigarette epidemic,” said Robin Koval, CEO and president of Truth Initiative. “This Is Quitting was developed with input from the very audiences we aim to reach—teens and young adults. We know they want to quit; we know what resonates with them, and we’re glad to be able to provide an effective resource to help them accomplish their goals to live vape-free. We’re grateful for the generous support from the CVS Health Foundation and are proud to contribute to a much-needed base of literature around the kinds of vaping cessation strategies that work.”
These findings show that text messaging is a scalable and cost-efficient approach to promote vaping abstinence among young adult e-cigarette users. Importantly for the field, the results of the clinical trial establish an effectiveness benchmark and begin to fill an important gap in understanding how to help young people quit e-cigarettes.
“This is Quitting is meeting a need for hundreds of thousands of young people through a channel they’re comfortable with and use every day,” said Dr. Amanda Graham, chief of innovations at Truth Initiative and lead author of the study. “The response to the program has been staggering since we launched it over two years ago with more than 500 young people enrolling each day. The strong and consistent results from this trial demonstrate that this program is providing the right support and information to help young people quit e-cigarettes.”
The double-blind, randomized clinical trial included 2,588 young adult e-cigarette users interested in quitting within 30 days. Participants were assigned to This is Quitting or a control intervention. Both groups received text messages asking about their e-cigarette use/abstinence. Participants randomized to the intervention group also received tailored, interactive support through This is Quitting to help them in their quitting journey. Outcome assessments were conducted at one and seven months. The trial also showed that This is Quitting participants were more satisfied compared to control participants across all items, with many saying that they received suggested quitting strategies that were new to them.
For more information about Truth Initiative, please visit truthinitiative.org. For more information on This is Quitting, please visit truthinitiative.org/thisisquitting.
BACKGROUND:
The clinical trial was conducted by Truth Initiative with oversight from Advarra Institutional Review Board. All program branding was removed in the trial to eliminate biases/exposure to marketing. Participants were recruited via social media ads. Incentivized text message assessments regarding e-cigarette use and abstinence were sent to all participants at 14 days post-randomization and monthly thereafter through 6 months All participants were compensated $5 via digital gift card per response (7 assessments total, maximum $35). These assessments were designed solely to maximize retention; they were not analyzed as outcomes.
Funding/Support: This trial was funded by Truth Initiative and the CVS Health Foundation. The CVS Health Foundation had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The latest news from Truth Initiative
Visit press centerWant support quitting? Join EX Program
By clicking JOIN, you agree to the Terms, Text Message Terms and Privacy Policy.
Msg&Data rates may apply; msgs are automated.